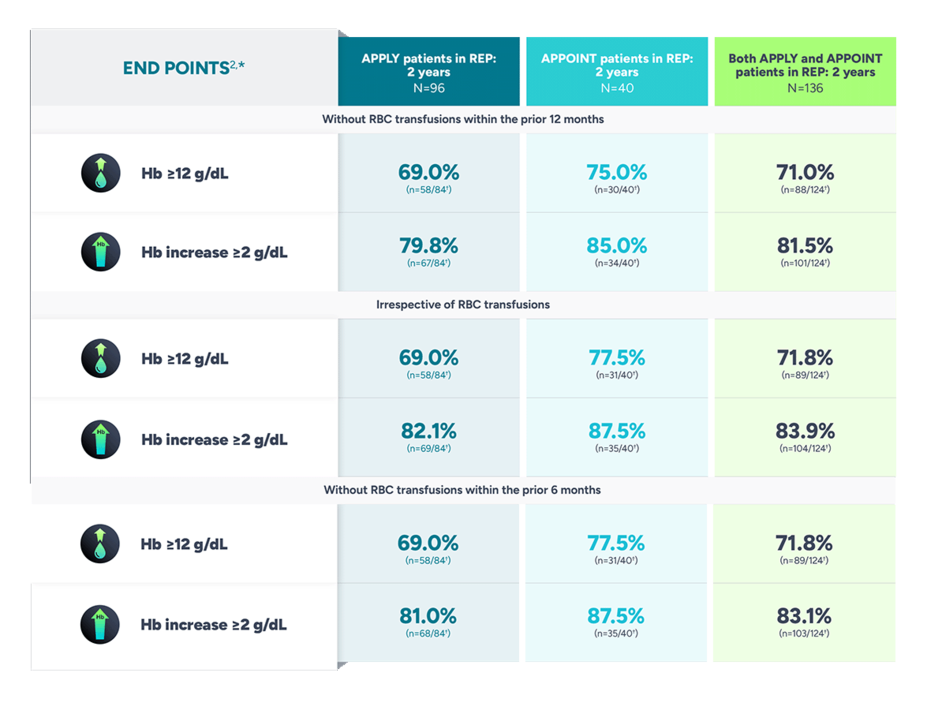
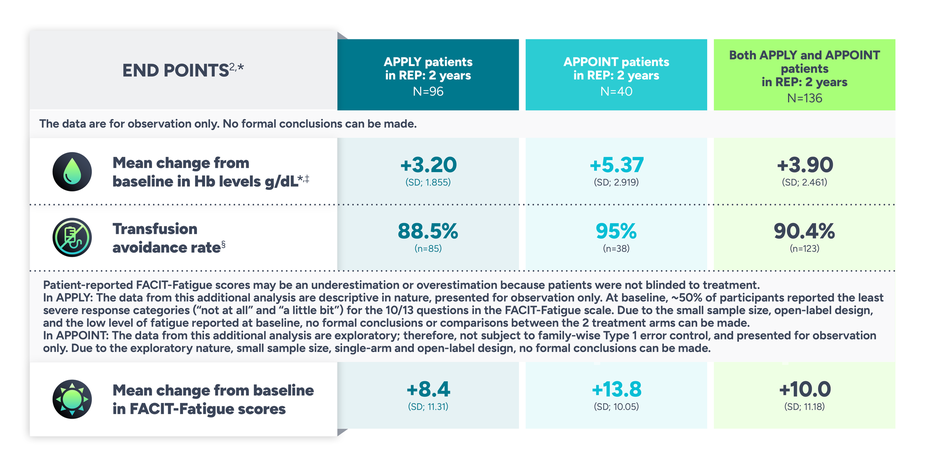
Explore the long-term data of FABHALTA through 2 years
Data from the rollover extension program (REP) of adults with PNH from APPLY and APPOINT through 2 years1
The data are for observation only. No formal conclusions can be made.
*Data from the REP are summarized descriptively.2
†Percentages at 2 years are based on the number of patients with Hb results at that time point.2
Additional end points
Mean LDH* remained <1.5 ULN through 2 years on FABHALTA2
In patients from APPLY: 33.00 U/L change from baseline (SD; 230.261); baseline: 273.84 (SD; 94.25)
In patients from APPOINT: -1399.18 U/L change from baseline (SD; 652.417); baseline: 1698.78 (SD; 683.33)
In patients from APPLY and APPOINT: -428.99 U/L change from baseline (SD; 789.05);‡ baseline: 692.94 (SD; 752.20)
*Data from the REP are summarized descriptively.2
‡Excludes values within 30 days post-transfusion.2
§The number of transfusions does not include the period Days 1-14 after starting LNP.2