APPULSE: A study of adults with PNH with Hb ≥10 g/dL who switched from a stable C5i regimen (eculizumab or ravulizumab) to FABHALTA1
Patients with baseline Hb ≥10 g/dL experienced an Hb increase after switching from a stable C5i regimen to FABHALTA
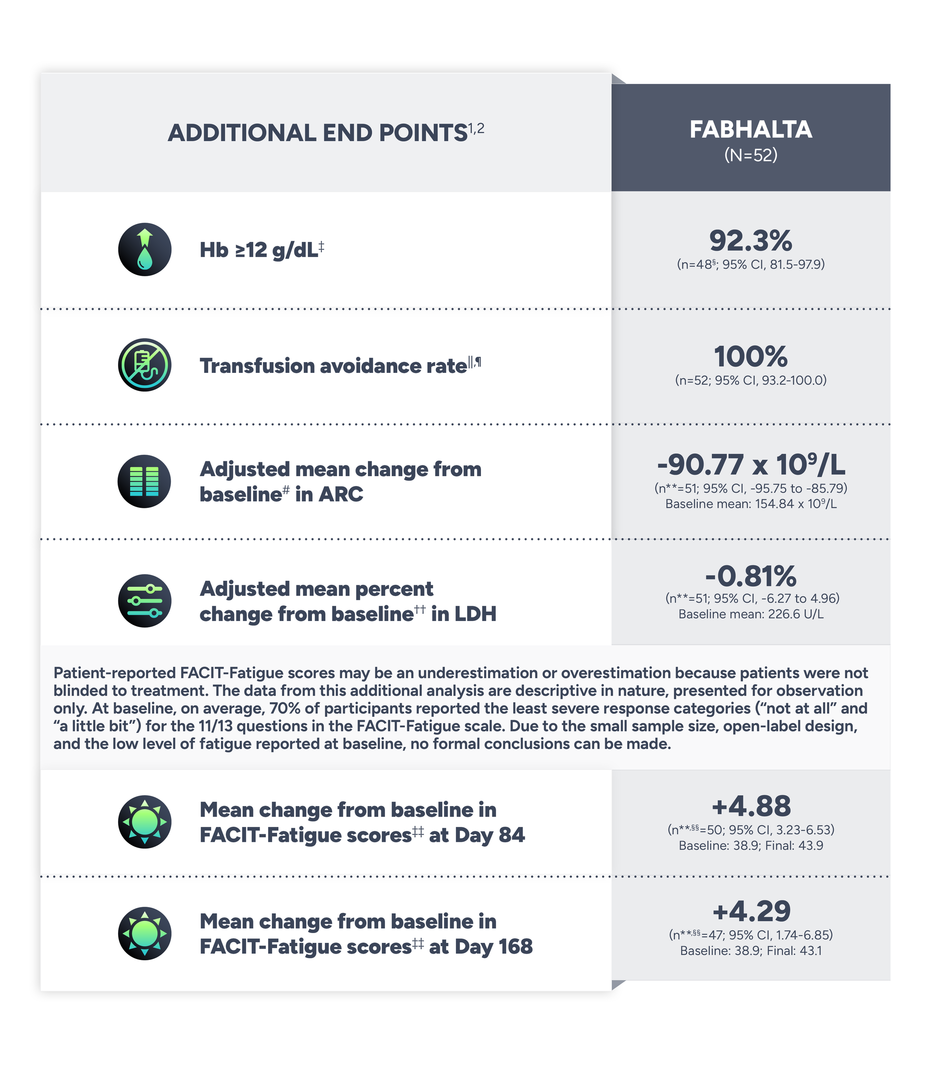
PRIMARY END POINT
The data are for observation only. No formal conclusions can be made.
*Mean of visits between Days 126 and 168 compared with baseline, defined as mean of Hb collected at screening (2 samples) and Day 1. Excludes values within 30 days post-transfusion.2
†The ‘n’ values reflect patients with non-missing values.2
Explore the data of FABHALTA in patients with Hb ≥10 g/dL after the 24-week treatment period
The data are for observation only. No formal conclusions can be made.
‡Assessed between visits Days 126 and 168 in the absence of RBC transfusions between Days 1 and 168, on 3 of 4 measurements taken at the visits occurring in the last 6 weeks.2
§Percentages are based on the number of patients with Hb results at that time point.2
||From 6 months to screening, no transfusions received. In the 12 to 6 months prior to the trial screening period, 3.8% (n=2/52) of patients received a transfusion.1
¶Transfusion avoidance response rate in APPULSE was defined as absence of administration of packed-RBC transfusions between Days 1 and 168.1
#Change from baseline as mean of visits between Days 126 and 168.2
**The ‘n’ values reflect patients with non-missing values.2
††Percentage change from baseline as mean of visits between Days 126 and 168.2
‡‡The Functional Assessment of Chronic Illness Therapy-Fatigue Scale (FACIT-Fatigue) is a 13-item questionnaire that assesses self-reported fatigue and its impact upon daily activities and function. The level of fatigue is measured on a 5-point Likert scale (in the study, 4=not at all fatigued to 0=very much fatigued), with 0 being the worst possible score and 52 the best.1,3
§§Baseline mean FACIT-Fatigue scores and adjusted mean change in FACIT-Fatigue scores at Day 84 and 168 were reported for 50 and 47 patients, respectively.1