Meet Kim:
An active and adventurous mother of 3 from South Carolina taking FABHALTA for PNH
Explore Kim's PNH journey with FABHALTA
57 years old | Diagnosed with PNH in 2021
Practices yoga, Pilates, and weightlifting
Loves traveling with her friends and family
Enjoys cooking and exploring new cuisines
Kim faced common challenges in her initial PNH treatment journey:
Low Hb levels, EVH, and managing her infusion schedule
Prior to diagnosis, Kim was experiencing shortness of breath and noticed unusual swelling in her ankles, which prompted her to visit her doctor
Follow-up bloodwork revealed that she had critically low Hb levels, leading to her hospitalization and diagnosis with PNH
For the next few years, Kim tried several treatments (including different C5is) but she did not respond as expected due to her EVH and was also looking for an alternative to her infusions
Kim and her doctor were at a crossroads, until she switched to FABHALTA:
Today, Kim is taking FABHALTA—and enjoying a new perspective on life with PNH:
BEFORE FABHALTA
Kim experienced below normal Hb levels on various treatments.
WITH FABHALTA
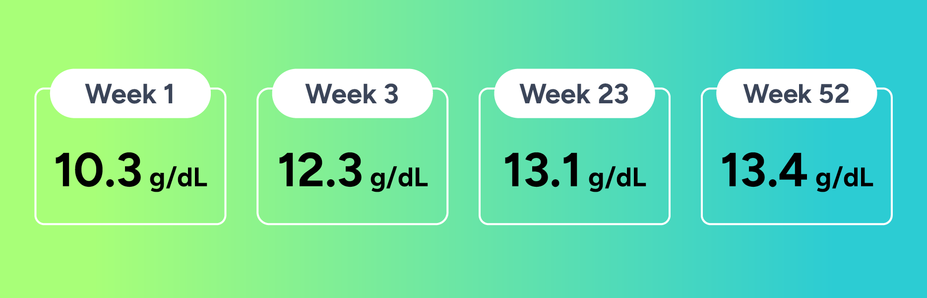
Since initiating treatment with FABHALTA in January of 2024, Kim shares that her Hb levels are “consistently in the 12s and 13s.”
Kim’s Hb levels with FABHALTA
Individual testing in clinical practice and results may vary.
Laboratory values provided by the patient.
APPLY was a 24-week, randomized,* open-label, active comparator–controlled, phase 3 trial to assess the efficacy and safety of switching to FABHALTA 200 mg twice daily compared with continuing on intravenous C5i therapy (US-approved and non–US-approved eculizumab or ravulizumab) in adults with PNH and residual anemia (mean Hb <10 g/dL) despite previous treatment with a stable regimen of C5i treatment for at least 6 months; 97 patients were randomized in an 8:5 ratio to either switch to FABHALTA 200 mg taken orally twice daily (n=62) or continue their C5i regimen (n=35: eculizumab, n=23; ravulizumab, n=12). The primary end points in the randomized period were the proportion of patients achieving Hb increase of ≥2 g/dL and the proportion of patients achieving Hb level of ≥12 g/dL,† both without the need for RBC transfusions.1,2,‡
RESPONSE RATES: 82.3% (n=51/62; 95% CI, 70.5-90.8) of patients on FABHALTA achieved an Hb increase of ≥2 g/dL§ from baseline in the absence of RBC transfusions‡ after 24 weeks vs 0% (n=0/35; 95% CI, 0-10.0) of patients on C5is (eculizumab or ravulizumab) (differenceII: 81.5%; 95% CI, 71.6-91.4; P<0.0001). 67.7% (n=42/62; 95% CI, 54.7-79.1) of patients on FABHALTA achieved a normalized¶ Hb of ≥12 g/dL§ in the absence of RBC transfusions‡ after 24 weeks vs 0% (n=0/35; 95% CI, 0-10.0) of patients on C5is (eculizumab or ravulizumab) (differenceII: 66.6%; 95% CI, 54.6-78.6; P<0.0001).1
SAFETY PROFILE OF FABHALTA: The adverse reactions reported in >5% of adults with PNH treated with FABHALTA vs C5is in APPLY (24-week treatment period) were1: headache (19% vs 3%), nasopharyngitis (16% vs 17%), diarrhea (15% vs 6%), abdominal pain (15% vs 3%), bacterial infection (11% vs 11%), nausea (10% vs 3%), viral infection (10% vs 31%), arthralgia (8% vs 3%), thrombocytopenia (6% vs 0%), dizziness (6% vs 0%), systemic hypertension (6% vs 0%), and lipid disorder (6% vs 0%).
*Randomization was stratified based on prior C5i treatment and transfusion history within the last 6 months.1
†Assessed between Days 126 and 168.1
‡Assessed between Days 14 and 168. Requiring RBCs refers to any patient receiving transfusions or meeting protocol-defined criteria.2
§Adjusted mean assessed between Weeks 18 and 24 (Days 126 and 168). Excludes values within 30 days post-transfusion.1
IIAdjusted difference in proportion.1
¶Normalization defined as meeting the primary end point of Hb ≥12 g/dL.2 Normal Hb levels vary but generally are between 12-16 g/dL for women and 13-18 g/dL for men.3
BEFORE FABHALTA
Her previous treatments only treated IVH, but Kim also has EVH.
WITH FABHALTA
Kim was excited to learn that FABHALTA addresses both drivers of hemolysis in PNH.
FABHALTA acts proximally in the alternative complement pathway to control both C3b-mediated extravascular hemolysis and terminal complement-mediated intravascular hemolysis.
BEFORE FABHALTA
After years of managing her infusion schedule, Kim was interested in a treatment without infusions.
WITH FABHALTA
With an oral option to manage PNH, Kim’s busy days are dedicated to travel, Pilates, and spending time with her family and friends.
Patients take 1 capsule (200 mg) twice daily and can be taken without regard to food.
Kim’s treatment experience changed when she and her doctor found FABHALTA
FABHALTA is the first and only oral monotherapy to help deliver comprehensive hemolysis control (IVH and EVH) in PNH.1

Meet Shonda
A C5i-experienced patient now taking FABHALTA