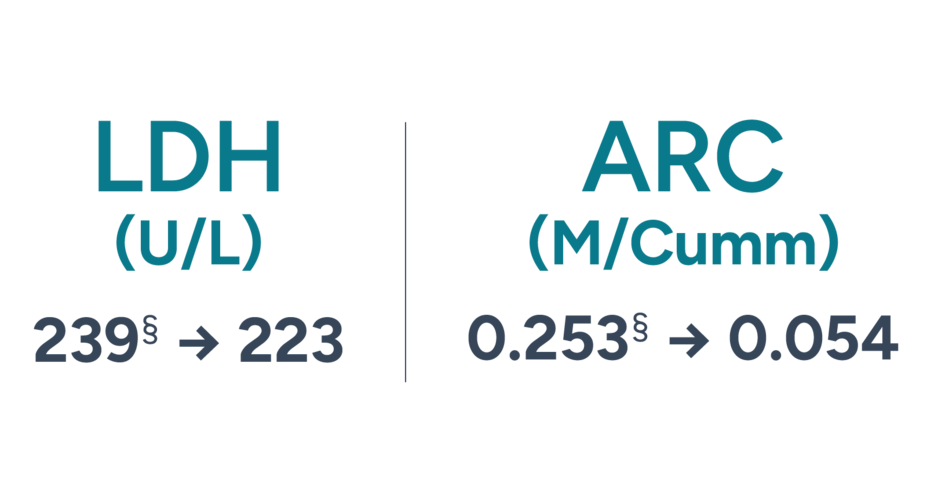Meet Shonda:
An accomplished horticulturist and devoted wife, mother, and grandmother, she‘s celebrating how far she‘s come with FABHALTA
For Shonda, stability wasn’t enough.
A switch to FABHALTA took her further
At the start of her PNH journey, Shonda was a shell of her former self
During the 6 years she spent searching for a diagnosis, Shonda struggled to get through each work day, gradually distancing herself from her loved ones and her favorite activities.
Today, Shonda is on FABHALTA and doing what she enjoys:
Leading exciting and challenging projects at work, spending time with her family at the lake, and tending to her vibrant garden.
From “Fine” to FABHALTA: Shonda’s switch from a C5i
Shonda’s Hb is now within a normal range on FABHALTA.*
Shonda experienced a 3.2 g/dL increase in Hb since starting FABHALTA.
These are 2 of the known biomarkers of IVH and EVH. Unlike C5 inhibitors, FABHALTA helps to control both of these types of hemolysis.1-3
Shonda has not required any RBC transfusions through 10 months of treatment with FABHALTA.‖
“Needing transfusions is very scary. So living my life without them up until this point has been incredible.”
*Normal Hb levels vary but generally are between 12-16 g/dL for women and 13-18 g/dL for men.4
†Based on patient-reported values as of April 2024.
‡Based on patient-reported values as of March 2025.
§Based on patient-reported values throughout duration of treatment.
‖Based on patient-reported values as of June 2025.
FABHALTA is the first and only oral monotherapy for adults with PNH, taken without infusions or injections.3
Shonda’s switch from a C5i took her treatment journey from “Fine” to FABHALTA.
FABHALTA is the first and only oral monotherapy to help deliver comprehensive hemolysis control (IVH and EVH) in PNH.3
APPLY was a 24-week, randomized,¶ open-label, active comparator–controlled, phase 3 trial to assess the efficacy and safety of switching to FABHALTA 200 mg twice daily compared with continuing on intravenous C5i therapy (US-approved and non–US-approved eculizumab or ravulizumab) in adults with PNH and residual anemia (mean Hb <10 g/dL) despite previous treatment with a stable regimen of C5i treatment for at least 6 months; 97 patients were randomized in an 8:5 ratio to either switch to FABHALTA 200 mg taken orally twice daily (n=62) or continue their C5i regimen (n=35: eculizumab, n=23; ravulizumab, n=12).3,5,**
PRIMARY END POINTS: The primary end points in the randomized period were the proportion of patients achieving Hb increase of ≥2 g/dL and the proportion of patients achieving Hb level of ≥12 g/dL,# both without the need for RBC transfusions.3,5,**
ADDITIONAL END POINTS: Additional end points of the APPLY study include adjusted mean change from baseline in ARC††, and adjusted geometric mean ratio to baseline in LDH‡‡ for FABHALTA vs C5is in patients with Hb<10 g/dL after the 24-week randomized treatment period.3,6
RESPONSE RATES FOR PRIMARY END POINTS: 82.3% (n=51/62; 95% CI, 70.5-90.8) of patients on FABHALTA achieved an Hb increase of ≥2 g/dL# from baseline in the absence of RBC transfusions** after 24 weeks vs 0% (n=0/35; 95% CI, 0-10.0) of patients on C5is (eculizumab or ravulizumab) (difference§§:81.5%; 95% CI, 71.6-91.4; P<0.0001). 67.7% (n=42/62; 95% CI, 54.7-79.1) of patients on FABHALTA achieved a normalizedIIII Hb of ≥12 g/dL# in the absence of RBC transfusions** after 24 weeks vs 0% (n=0/35; 95% CI, 0-10.0) of patients on C5is (eculizumab or ravulizumab) (difference§§: 66.6%; 95% CI, 54.6-78.6; P<0.0001).1,5
RESPONSE RATES FOR ADDITIONAL END POINTS: Adjusted mean change from baseline in ARC†† for patients on FABHALTA (n=62) was -116 x 109/L (n=62; 95% CI, -127 to -105, baseline mean: 193 x109/L) vs patients on C5is (eculizumab or ravulizumab) (n=35) was 0 x 109/L (n=35; 95% CI, -13 to 14, baseline mean: 191 x109/L) with an adjusted mean difference of -116 x 109/L; 95% Cl, -132 to -100; P<0.0001. Adjusted geometric mean ratio to baseline in LDH‡‡ for patients on FABHALTA (n=62) was 0.96 (n=62, baseline mean: 269 U/L) vs patients on C5is (eculizumab or ravulizumab) (n=35) was 0.97 (n=35, baseline mean: 273 U/L). No statistically significant difference in LDH was seen between FABHALTA and C5is. The data from this additional analysis are descriptive in nature, presented for observation only. No formal conclusions or comparisons between the 2 treatment arms can be made.3,6
SAFETY PROFILE OF FABHALTA: The adverse reactions reported in >5% of adults with PNH treated with FABHALTA vs C5is in APPLY (24-week randomized treatment period) were3: headache (19% vs 3%), nasopharyngitis (16% vs 17%), diarrhea (15% vs 6%), abdominal pain (15% vs 3%), bacterial infection (11% vs 11%), nausea (10% vs 3%), viral infection (10% vs 31%), arthralgia (8% vs 3%), thrombocytopenia (6% vs 0%), dizziness (6% vs 0%), systemic hypertension (6% vs 0%), and lipid disorder (6% vs 0%).
Serious adverse reactions were reported in 2 (3%) patients with PNH who received FABHALTA. They included pyelonephritis, urinary tract infection, and COVID-19
Rash was reported in 2 patients (3%)
Of the 37 FABHALTA-treated patients who had normal platelet counts at baseline, 43% experienced any grade thrombocytopenia during the randomized treatment period
Three FABHALTA-treated patients experienced decreased platelets that worsened to grade ≥3 from baseline (1 patient with normal platelets that worsened to grade 4; 1 patient with baseline grade 1 that worsened to grade 4; and 1 patient with baseline grade 3 that worsened to grade 4)
No patient discontinued FABHALTA or C5is due to an adverse reaction during the 24-week randomized treatment period. One patient discontinued FABHALTA due to pregnancy.5
¶Randomization was stratified based on prior C5i treatment and transfusion history within the last 6 months.3
#Assessed between Days 126 and 168.3
**Assessed between Days 14 and 168. Requiring RBCs refers to any patient receiving transfusions or meeting protocol-defined criteria.5
††Mean change from baseline in ARC (109/L) assessed between Days 126 and 168. Values include post-transfusion data.3,6
‡‡Mean ratio to baseline in LDH assessed between Days 126 and 168.6
§§Adjusted difference in proportion.3
IIIINormalization defined as meeting the primary end point of Hb ≥12 g/dL.5 Normal Hb levels vary but generally are between 12-16 g/dL for women and 13-18 g/dL for men.4
APPULSE was a 24-week, single-arm, open-label, multicenter, phase 3b trial to evaluate the efficacy and safety of switching to iptacopan 200 mg twice daily in adults with PNH who had achieved Hb levels ≥10 g/dL¶¶ in response to a stable regimen of anti-C5 antibody treatment (eculizumab or ravulizumab) for at least 6 months and had remained transfusion-free during that period. Patients (N=52) were enrolled following an 8-week screening to confirm eligibility, including transfusion history and vaccination status. All received oral iptacopan 200 mg twice daily for 24 weeks.7
PRIMARY END POINT: The primary end point was the adjusted mean change from baseline in Hb.7,8,##
ADDITIONAL END POINTS: Additional end points of the APPULSE study include the number of patients with Hb ≥12 g/dL assessed between visits Days 126 and 168 in the absence of RBC transfusions between Days 1 and 168***, adjusted mean percent change from baseline††† in LDH in patients with Hb ≥10 g/dL after the 24-week treatment period, transfusion avoidance rate‡‡‡,§§§ in patients with Hb ≥10 g/dL after the 24-week treatment period, and adjusted mean change from baselineIIIIII in ARC in patients with Hb ≥10 g/dL after the 24-week treatment period.7,8
RESPONSE RATES FOR PRIMARY END POINT: The adjusted mean change from baseline in Hb## for the patients studied was +2.07 g/dL ((n¶¶¶=51/52) (95% CI, 1.80-2.33) (mean [SD] baseline Hb levels [g/dL]: 11.87 [1.32]). The data are for observation only. No formal conclusions can be made.7,8
RESPONSE RATES FOR ADDITIONAL END POINTS: The number of patients with Hb ≥12 g/dL assessed between visits Days 126 and 168, in the absence of RBC transfusions between Days 1 and 168***, was 92.3% (n=48/52###) (95% CI, 81.5-97.9). In patients on FABHALTA (N=52) with Hb ≥10 g/dL after the 24-week treatment period, the adjusted mean percent change from baseline††† in LDH was -0.81% (n¶¶¶=51; 95% CI, -6.27 to 4.96, baseline mean: 226.6 U/L), the transfusion avoidance rate‡‡‡,§§§ was 100% (n=52; 95% CI, 93.2-100.0), and the adjusted mean change from baselineIIIIII in ARC was -90.77 x 109/L (n¶¶¶=51; 95% CI, -95.75 to -85.79, baseline mean: 154.84 x 109/L). The data are for observation only. No formal conclusions can be made.7,8
SAFETY PROFILE OF FABHALTA: The adverse reactions reported in >5% of adults with PNH treated with FABHALTA in APPULSE (24-week treatment period) were9: headache (17.3%), nasopharyngitis (17.3%) , viral infection (13.5%), diarrhea (11.5%), nausea (11.5%), bacterial infection (9.6%), lipid disorder (7.7%), thrombocytopenia (5.8%). A serious adverse reaction (bacterial pneumonia) was reported in 1 patient (2%).
ADDITIONAL END POINTS: During the 24-week treatment period, no clinical breakthrough hemolysis**** or major adverse vascular events (MAVEs)†††† were observed in patients on FABHALTA. The data are for observation only. No formal conclusions can be made.7
One patient discontinued FABHALTA due to an adverse reaction (palpitations)9
¶¶Mean Hb >10 g/L over a period of 6 months before screening visit and confirmed by 2 different samples during the screening period.7
##Mean of visits between Days 126 and 168 compared with baseline, defined as mean of Hb collected at screening (2 samples) and Day 1. Excludes values within 30 days post-transfusion.8
***Assessed on 3 of 4 measurements taken at the visits occurring in the last 6 weeks.8
†††Percentage change from baseline as mean of visits between Days 126 and 168.8
‡‡‡From 6 months to screening, no transfusions received. In the 12 to 6 months prior to the trial screening period, 3.8% (n=2/52) of patients received a transfusion.7
§§§Transfusion avoidance response rate in APPULSE was defined as absence of administration of packed-RBC transfusions between Days 1 and 168.7
IIIIIIChange from baseline as mean of visits between Days 126 and 168.8
¶¶¶The ‘n’ values reflect patients with non-missing values.8
###Percentages are based on the number of subjects with Hb results at that time point.8
****Rate of occurrence through Day 168. Summary measure was occurrences per year.7
††††The definition of MAVEs included: acute peripheral vascular occlusion, amputation, cerebrovascular accident, cerebral venous occlusion, dermal thrombosis, gangrene, hepatic/portal vein thrombosis, mesenteric/visceral arterial or vein thrombosis or infarction, myocardial infarction, pulmonary embolus, renal arterial or vein thrombosis, thrombophlebitis/deep vein thrombosis, transient ischemic attack, and unstable angina.7

Meet Kim
A C5i-experienced patient now taking FABHALTA